Hydrogen as an Energy Carrier in the Future – The Petroleum of Tomorrow?

In 2021, the consumption of raw materials in Germany amounted to 95.5 million tons [1]. In this context, crude oil is primarily used as an energy source. This is understood to mean substances or other forces that are capable of performing work in the physical sense. [2] Petroleum can thus be used in the first step for storage, then for transportation, and finally for the production of energy. However, natural petroleum reserves are limited and, furthermore, extraction and use represent an extreme burden on the environment and climate. The future viability of this energy source is therefore questionable. Consequently, an alternative is needed to adequately fill the gap in the market. An energy carrier that is similar to petroleum and is used both as an "industrial raw material" and as a "material basis for the production of synthetic energy carriers" [3] is hydrogen.
Hydrogen can store energy for months and is not toxic, corrosive, or radioactive [4]. The potential seems great, as hydrogen plays a key role in decarbonization. That is, the "process of reducing 'carbon intensity,' thereby reducing the amount of greenhouse gas emissions produced by burning fossil fuels" [5]. Decarbonization is a key challenge of the 21st century. Also on the part of the German government, hydrogen technologies are seen as having a promisingly high potential. The national hydrogen strategy states: "In addition to the benefits for the climate and security of supply, hydrogen technologies also have the potential to create many future-proof jobs and a global market worth billions" [6].
Hydrogen as an energy carrier - illustrating the potential for sustainability
The enormous power of hydrogen can be illustrated in a very simplified way using a simple experiment, the oxyhydrogen explosion. In this experiment, a balloon is filled with hydrogen, sealed and, under appropriate safety precautions, caused to explode by approaching a burning match [7]. The enormous force of the hydrogen is made clear by the loud bang. It is also striking that only water remains as the final product [8]. The oxyhydrogen explosion thus illustrates, in a highly simplified and theoretical way, the high potential of hydrogen as an energy carrier. The fact that only hydrogen remains as a product means that no harmful by-products or waste products are produced.
Extraction and production of hydrogen
Hydrogen is the most common chemical element in nature. As a rule, it is found exclusively bonded with other elements. Hydrogen is therefore a secondary energy carrier, i.e. an energy carrier that does not come directly from nature but must first be converted [9]. At normal temperature, hydrogen is gaseous [10]. Hydrogen is produced in four different common ways. The respective way and the corresponding process give the obtained form of hydrogen its designation. A distinction is made between gray, blue, turquoise and green hydrogen. In view of the future viability of hydrogen as an energy carrier, the latter process is the most suitable. The individual production processes are explained below:
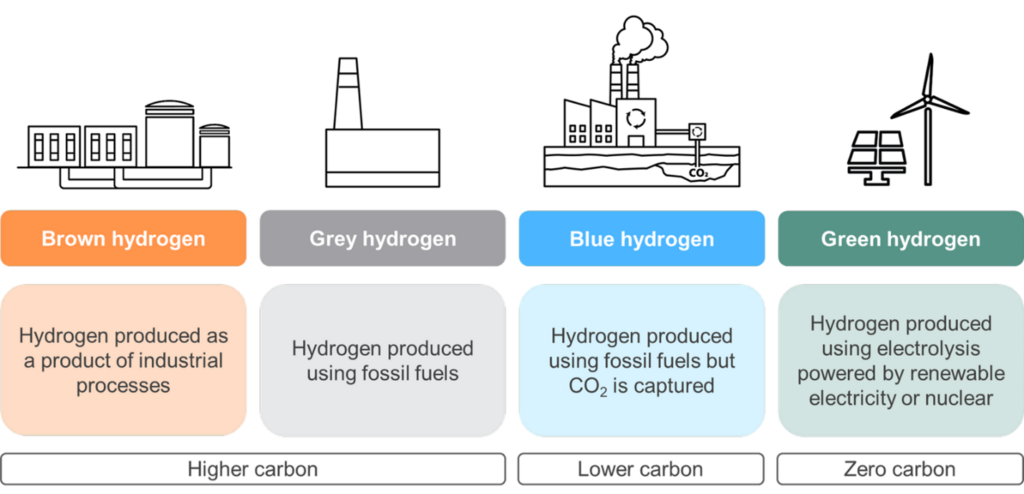
Illustration: Hydrogen. Bluesource: https://www.bluesource.com/blog/green-blue-grey-hydrogen/
Gray hydrogen
In this process, hydrogen is split off from an existing fossil carbon, usually natural gas. This is done under heat by a process known as steam reforming. In the process, natural gas reacts with water vapor. In addition to the hydrogen produced, however, carbon dioxide is also produced, which escapes into the atmosphere [11]. Gray hydrogen is thus not considered sustainable due to its waste product.
Blue hydrogen
Blue hydrogen is also produced based on a key fossil resource using steam reforming. At this point, unlike gray hydrogen, a carbon capture and storage (CCS) strategy is used to capture and store CO₂ emissions [11]. Thus, this type of hydrogen production is already considered CO₂-neutral [10], i.e., with no impact on atmospheric carbon dioxide concentrations, since the byproducts are appropriately bundled.
Turquoise hydrogen
Turquoise hydrogen is produced by the thermal cracking of methane. The high temperatures cause bond breaking of the chemical compounds [12]. This process is referred to as pyrolysis. Unlike gray and blue hydrogen, no gaseous CO₂ is produced, but a solid carbon [11]. To guarantee the sustainability of this process, it must be ensured that renewable energies are used. In addition, the solid carbon must be permanently sequestered.
Green hydrogen
Green hydrogen is produced by decomposing water into oxygen and hydrogen. Electric current is used in the process. This process is called electrolysis. If the electricity used is of sustainable origin, the hydrogen produced is considered green [13]. Accordingly, the basis for a sustainable process is renewable energy. In addition, no further waste products are produced. Green hydrogen is thus almost climate-neutral and is well suited as an energy carrier. However, this also requires sufficient sustainable electricity.
Price development
As explained, the production of green hydrogen is based on electrolysis technology. However, this production process is still under development and is not yet fully efficient. At present, only part of the energy can be converted. About 25 % is lost in the form of waste heat. Today, "with this new technology, an industrial level with corresponding cost degression is not yet possible" [14]. Due to the level of effort and the not yet fully developed production process, green hydrogen is only produced in small quantities. High production costs, which directly affect the price of the final product, are the result. Consequently, the number of potential customers also decreases. The price is also volatile due to the enormous amount of sustainable electricity required. On the one hand, there is a lack of capacity to produce exclusively in Germany, and on the other hand, normal weather fluctuations influence the price development.
Nevertheless, the approaches of the current modes of operation are promising and offer great potential, which is supported by appropriate subsidies. Predicted price developments reflect this. Should the technology increase in efficiency, the price will fall. Production costs would then be lower, making the product, green hydrogen more attractive to buyers and consumers. Consequently, it can then be produced more cheaply and in larger quantities.
Hydrogen strategy in Germany
"Despite the Corona pandemic, we must not lose any more time on the subject of hydrogen" [15], clarified the former Federal Minister of Research, Anja Karliczek at the beginning of 2020, referring to the enormous potential to replace petroleum and other fossil fuels in the long term and sustainably. In order to exploit this accordingly, research and revision of existing strategies to produce green hydrogen are needed.
The German government is promoting this future technology as part of its national hydrogen strategy. The aim is to "reduce CO₂ emissions in the industrial, transport and energy sectors on the basis of hydrogen technology" [6]. The Federal Ministry of Research supports initiatives that optimize hydrogen. Real laboratories of the energy turnaround, which are available as test rooms for innovation and regulation, are funded by the Federal Ministry of Economics and Climate Protection, for example. The Federal Ministry of Transport promotes the use of hydrogen in transport through the National Hydrogen and Fuel Technologies Innovation Program.
Hydrogen as a key technology
Hydrogen is almost infinitely available in nature [16] and causes almost no greenhouse gas emissions during production. "The technology can save about 560 million tons of CO₂ per year by 2050" [17], according to a forecast published by the EU Parliament. The area of application is also broad. In both aviation and heavy-duty transport, as well as in shipping, green hydrogen offers a sustainable fuel technology with virtually no alternatives, unlike other sustainable technologies such as electric power.
For companies that specialize in precisely this area over the long term, a high profit margin can be realistically predicted. The increasing demand can be confirmed, among other things, by a study conducted by the auditing and consulting firm PwC. Here, rising demand is expected in all scenarios examined, and with regard to climate protection ambitious scenarios, even significantly stronger growth [18].
Hydrogen as the energy carrier of the future
In summary, green hydrogen cannot yet be considered a mature technology. The production process by electrolysis is not efficient enough to be successful on the free market. In addition, international cooperation is needed to provide the required amounts of renewable energy. Nevertheless, the existing approaches promise a lot of potential. This is evident from market analyses and broad support programs. Furthermore, strategies to reduce the costs of electrolysis are available, for example by the Fraunhofer Institute for Solar Energy Systems ISE [19]. Provided that the production processes are revised, green hydrogen could thus be a reliable and, above all, sustainable alternative to petroleum, provided that production processes are revised.
____________________________________________________________________________________________________________
[1] Cf. Statista: Verbrauch von Erdöl in Deutschland bis 2021. In https://de.statista.com/statistik/daten/studie/36171/umfrage/verbrauch-von-erdoel-in-deutschland-seit-1990/
[2] Prof. Dr. Hans-Dieter Haas, Gabler Wirtschaftslexikon: Energieträger. In https://wirtschaftslexikon.gabler.de/definition/energietraeger-36691
[3] Institut für angewandte Ökologie: Wasserstoff sowie wasserstoffbasierte Energieträger und Rohstoffe. In https://www.oeko.de/fileadmin/oekodoc/Wasserstoff-und-wasserstoffbasierte-Brennstoffe.pdf
[4] Cf. e-Mission: Umwelttechnologien, Wasserstoff. In https://platform.e-mission.de/de/courses/e-course-4/e-mod-1?sec=e-sec-15
[5] Cf. TwI Was ist Dekarbonisierung? In https://www.twi-global.com/locations/deutschland/was-wir-tun/haeufig-gestellte-fragen/was-ist-dekarbonisierung
[6] Cf. Bundesregierung, Energie und Klimaschutz – Wasserstoffstrategie der Bundesregierung „Warum gilt Wasserstoff als Energieträger der Zukunft.“ In https://www.bundesregierung.de/breg-de/themen/klimaschutz/wasserstoff-technologie-1732248
[7] Cf. Seilnacht: Knallgasexplosion, Wasserstoffballon in https://www.seilnacht.com/versuche/experih2.html
[8] Cf. Klima:neutral: Wasserstoff als Technologie der Zukunft? In https://www.youtube.com/watch?v=Bng_QFTd6MQ
[9] Cf. SFC Energy: Sekundärenergie hat viele Facetten. In https://www.sfc.com/glossar/sekundaerenergie/
[10] Cf. Bundesregierung, nationale Wasserstoffstrategie Wasserstoffstrategie der Bundesregierung „Warum gilt Wasserstoff als Energieträger der Zukunft“ in https://www.bundesregierung.de/breg-de/themen/klimaschutz/wasserstoff-technologie-1732248
[11] Cf. FfE München. Beitragsreihe Wasserstoff: Wie wird Wasserstoff Produziert? In https://www.ffe.de/veroeffentlichungen/beitragsreihe-wasserstoff-wie-wird-wasserstoff-produziert/
[12] Cf. Chemie.de: Pyrolyse in https://www.chemie.de/lexikon/Pyrolyse.html
[13] Cf. Bundesministerium für Wirtschaft und Klimaschutz: Was ist eigentlich grüner Wasserstoff? In https://www.bmwi-energiewende.de/EWD/Redaktion/Newsletter/2020/07/Meldung/direkt-erklaert.html
[14] ASUE: Grau, Blau oder Grün: Was kostet der Wasserstoff? in https://asue.de/aktuelles_presse/kosten_von_wasserstoff_hydex
[15] Cf. Ecomento (DE) Forschungsministerin drängt zu Eile bei Wasserstoffstrategie der Bundesregierung, Spiegel, 06.05.2020 in https://ecomento.de/2020/05/06/wasserstoffstrategie-der-bundesregierung-weiter-strittig/
[16] Cf. Ingeneur.de: Ist Wasserstoff endlich sauber und wirtschaftlich herstellbar? In https://www.ingenieur.de/technik/fachbereiche/energie/ist-wasserstoff-endlich-sauber-wirtschaftlich-herstellbar/
[17] Cf. e-Mission Glossar, Wasserstoff, Referenz zu EU-Parlament 19.05.2019 in https://platform.e-mission.de/de/courses/e-course-4/e-mod-1?sec=e-sec-iq7
[18] Cf. Pw.C Studie 2021: Der Markt für Wasserstoff entwickelt sich dynamisch, Chance zur Dekarbonisierung: Grüner Wasserstoff als Motor der Energiewende in https://www.pwc.de/de/energiewirtschaft/wasserstoff-ein-essentieller-baustein-der-energiewende/chance-zur-dekarbonisierung-gruener-wasserstoff-als-motor-der-energiewende.html
[19] „Die Forscher entwickeln neue Membranmaterialien, verlängern die Lebensdauer der Zellen durch eine Anti-Korrosions Beschichtung und führen entsprechende Lebensdauertests durch“ Cf. Fraunhofer-Gesellschaft: Wasserstoff – so bleiben wir mobil. In https://www.fraunhofer.de/de/forschung/aktuelles-aus-der-forschung/wasserstoff-so-bleiben-wir-mobil/herstellung-gruener-wasserstoff.html